|
(S-8A-4) Controlling the Nuclear Reaction
|
|
Magnetic Fields Optional: Quantum Physics (and 6 more)---------- our Galactic Center of Atoms and Nuclei (first of 5 linked sections) |
. 4. Controlling the Nuclear ReactionIt takes elaborate technology and design to get a nuclear chain reaction going. At the same time, the rate of fission cannot get too high. If more than 1 neutron per fission initiates another fission event, the temperature will gradually rise. The energy release is never fast enough for the reactor to explode like a bomb (one advantage of using thermal neutrons) but if the reaction grows out of control, it may quickly destroy the reactor.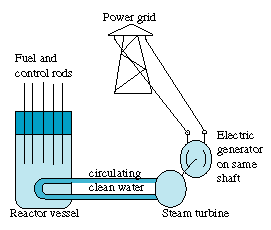 [Figure 5 -- Nuclear Power Station]
[Figure 5 -- Nuclear Power Station]
Control is maintained by control rods of a material such as the metal cadmium, which has a high "absorption cross section" for neutrons. The rods are automatically pushed deeper into the reactor to reduce the rate of fission, or pulled out to maintain or increase it. The fact which allows control is the existence of delayed neutrons. About 98% of the neutrons released in a fission are prompt neutrons, released very quickly, faster than the reaction time of automatic control machinery. However, 2% are delayed neutrons, which provide a very narrow margin for reactivity control. Reactors need to stay on the 2% margin between a fizzle and runaway fission. It is very small margin, and because of its narrowness, any power reactor has multiple independent safety devices |
|
In case of an emergency, an emergency shut-down (a "scram") automatically pushes or drops the rods in all the way, as well as extra rods for emergency use, usually withdrawn. The chain reaction then stops immediately, but not the radioactive decay of fission fragments. The energy these release is much less than that of the fission process, but in the hours after shutdown enough heat is still produced to melt or damage parts of the reactor ("nuclear meltdown") so the flow of cooling water must be maintained. On 28 March 1979 the power reactor at Three Mile Island in Pennsylvania encountered a problem and shut down automatically, but because operators misinterpreted the behavior of the reactor and shut down safety controls which provide cooling in an emergency, it suffered a partial meltdown. In the US and most countries, reactors are enclosed in a thick concrete containment building, so that even if meltdown occurred and contaminated fission products escaped the reactor itself (not the case at Three Mile Island), they are kept from spreading Operator error was also the cause of a reactor accident at Chernobyl on 25 April 1986. One of the reactors in a power station supplying Kiev, the capital of the Ukraine, went "prompt critical," with its chain reaction sustained by the uncontrollable prompt neutrons alone. It had a graphite core, and the sudden heat release blew off the top of its enclosure. The core then caught fire, generating a smoke plume laced with radioactive fission products, contaminating a wide area around the station, which was evacuated (and remains so), and also spreading radioactive contamination over parts of Europe. Meltdown also occurred reactors of the Fukushima incident in Japan in March 2011, when a major earthquake and its tsunami wave disabled the cooling of reactors in a nuclear power station. Breeder ReactorsThe first commercial power reactor, a relatively small one, started operating in 1957 near Shippingport, outside Pittsburgh, Pennsylvania. It originally used a conventional fuel cycle based on 235U and slowed-down ("thermal") neutrons. In 1977 it was however restructured to successfully "breed" thorium into 233U (reference #16). Power generation ended in 1982, after a run of 25 years, and the reactor was successfully decomissioned and buried in a distant site in Washington State Breeder reactors based on uranium are difficult to design and maintain, because the conversion of 238U to plutonium is more efficient with fast neutrons (also used in nuclear bombs). They cannot be cooled by water (which slows down neutrons) but operate at high temperatures and are cooled by a metal above its melting point, e.g. liquid sodium. Some such "fast breeders" were built and ran successfully, but so far have played only a minor role in power generation. Tidbit: The uranium mines of Gabon, Africa, have been supplying the French power system with nuclear fuel. In 1972 it was discovered that some uranium deposits from Oklo, Gabon, were slightly depleted in 235U, and contained an unusual variety of isotopes which might have come from nuclear fission (reference #14). It is believed that about 1.5 billion years ago, when the concentration of 235U was higher (its half-life is about 0.8 billion years), a natural fission process was sustained in some of the deposits, for a long time. It was caused by water leaking into the deposit and forming a natural moderator. The process was probably cyclical--heat generated by fission would drive out the water and stop the reaction, until fresh water entered again. Problems(answers in section S-8A-5)(1) Why is the nuclear power industry interested in elements such as deuterium (2H), carbon (12C), cadmium, Thorium (232T), Uranium (238U), (235U) and (233U),, Plutonium (239Pu), (2) Compile a glossary, defining briefly in alphabetical order in your own words: Breeder reactor, Cadmium, Chernobyl accident, Containment building, Control rods, Fast neutrons, Meltdown, Oklo phenomenon, Prompt critical nuclear reactor, Thorium cycle, Three Mile Island accident, Final Note
The first controlled nuclear reaction (image above) was achieved on 2 December 1942. The reactor was a near-spherical "pile" of pure graphite (carbon) bricks, in which cans of uranium oxide were embedded at fixed intervals, and holes were also left for control rods. It was located in a closed space under stadium seating (later torn down) at the University of Chicago, and the project was led by the Italian physicist (and Nobel laureate) Enrico Fermi. After a successful chain reaction was achieved (kept at low level, since no cooling was provided), Arthur Compton, one of the leaders of the project, reported by telephone to James Conant in Washington, chairman of the national Defense Research Committee . The project was secret, so he had to improvise. He said (from (reference #15, abbreviated):
Conant replied: "Were the natives friendly?" Compton: "Everyone landed safe and happy"
References#1 Overview of discoveries related to atoms and nuclei: http://www.phy6.org/stargaze/Ls7adisc.htm #2. Ions in water solutions, http://www.phy6.org/Education/whposion.html #3 Electrons "boiled off" a hot wire in vacuum, http://www.phy6.org/Education/welect.html #4 About electromagnetic radiation, http://www.phy6.org/stargaze/Sun5wave.htm #5 Quantum phenomena, http://www.phy6.org/stargaze/Q1.htm and the 7 sections Q2 ...Q7 that follow it. #6 "Spectral lines" of various elements, emitted when they descend from a high energy level to a lower one, http://www.phy6.org/stargaze/Sun4spec.htm #7 Why planets have negative energy, http://www.phy6.org/stargaze/Skepl2nd.htm #8 http://www.phy6.org/stargaze/Sun7enrg.htm (near the end) #9 Units of particle energies, http://www.phy6.org/Education/wenpart1.html #10 (a) Section on nuclear fission in "Hyperphysics" by Rod Nava, http://hyperphysics.phy-astr.gsu.edu/Hbase/nucene/u235chn.html (b) Also, on the curve of binding energy http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/nucbin.html #11 The photon, http://www.phy6.org/stargaze/Sun5wave.htm, (at its end). #12 "Nuclear Power" http://www.phy6.org/stargaze/Snuclear.htm Related site on nuclear weapons, http://www.phy6.org/stargaze/Snucweap.htm Also on the Sun's energy. http://www.phy6.org/stargaze/Sun7enrg.htm #13 Nuclear power in space, http://www.eoearth.org/article/Nuclear_reactors_for_space #14 The natural reactor at Oklo, http://en.wikipedia.org/wiki/Oklo_phenomenon #15 "The Making of the Atomic Bomb " by Richard Rhodes, 886 pp.,Simon and Schuster 1988. "Nuclear Renewal," is a short book about nuclear energy by the same author, reviewed at http://www.phy6.org/outreach/books/NuclEnrg.htm #16 "If Nuclear Power Has a More Promising Future ..." by Leslie Allen, "Washington Post Magazine" Sunday supplement, 2 August 2009 (posted here).
|
